Quinet et. al, Mol. Cell 2020
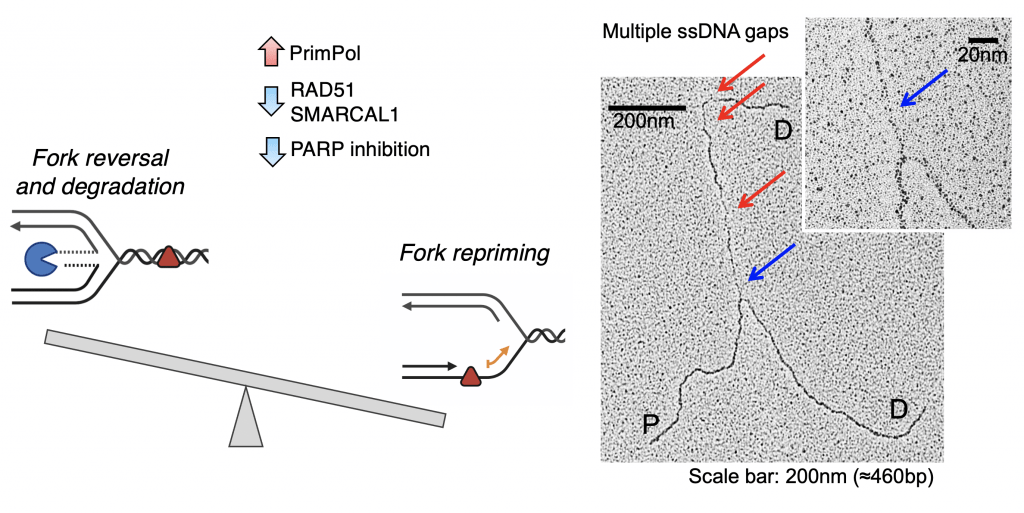
Most of the previous studies, including our own work, investigated how replication is perturbed by DNA-damaging chemotherapeutics following a single-dose treatment, neglecting the fact that patients are treated with multiple doses in a clinical setting. We began to more broadly consider how the replication stress response to DNA damaging chemotherapeutics changes after treatment with multiple drug doses. Using a novel multiple dose approach, we discovered that cells restrain fork reversal when reversed forks cannot be adequately protected by BRCA proteins. In particular, we found that BRCA1-deficient cells adapt to treatment with multiple doses of platinum-based compounds by suppressing fork reversal and promoting PRIMPOL-repriming as an alternative strategy to cope with the drug-induced DNA lesions. This effect is generalizable to other conditions of impaired fork reversal (such as loss of the SMARCAL1 translocase or PARP inhibition) and suggests a new strategy to modulate DNA-damaging chemotherapeutic sensitivity by targeting the PRIMPOL pathway. Our next goal is to expand this analysis to different DNA damaging chemotherapeutics and define whether different adaptive response mechanisms are activated at later time points after drug treatment, depending on the nature of the DNA lesion.
You must be logged in to post a comment.