Berti et. al, Nat. Struct. Mol Biol. 2013
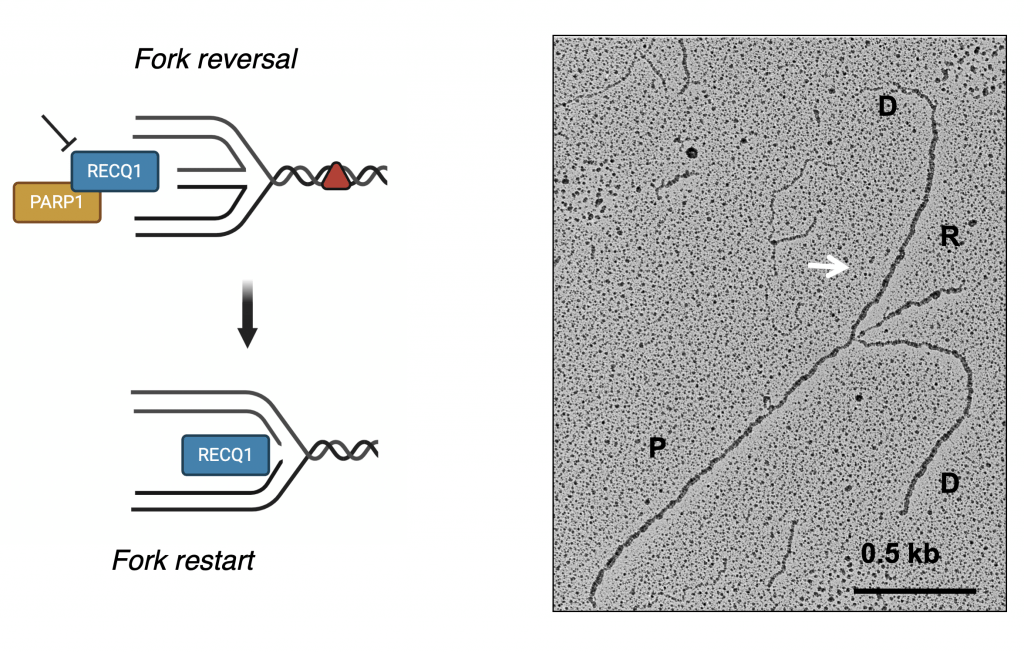
Our long-term goal is to define the mechanisms that ensure error-free processing of stalled or damaged replication forks, and to understand how to target these mechanisms for the development of new and more effective strategies for cancer treatment. Specifically, our lab made substantial contributions toward the understanding of the mechanism of replication fork reversal that allows replication forks to reverse their course to cope with DNA lesions. We uncovered a key role of the human RECQ1 helicase in the restart of reversed replication forks. We also found that the fork restart function is regulated by poly(ADP-ribose) polymerase (PARP1), which suppresses RECQ1 activity until the damage is repaired, providing the first insight into the molecular steps that drive the timely resolution of reversed replication forks. Shortly thereafter, we identified a second human DNA2- and WRN-dependent mechanism of reversed fork processing and restart. We also found that depletion of the central recombinase RAD51 antagonizes this mechanism, presumably by preventing reversed fork formation. Collectively, our work provided the first mechanistic insight into how replication forks reverse and restart as a pivotal response to treatment with DNA damaging chemotherapeutics, and offers new molecular perspectives to potentiate current DNA-damaging chemotherapeutic regimens by targeting fork reversal.
You must be logged in to post a comment.