Lemacon et. al, Nat. Comm. 2017
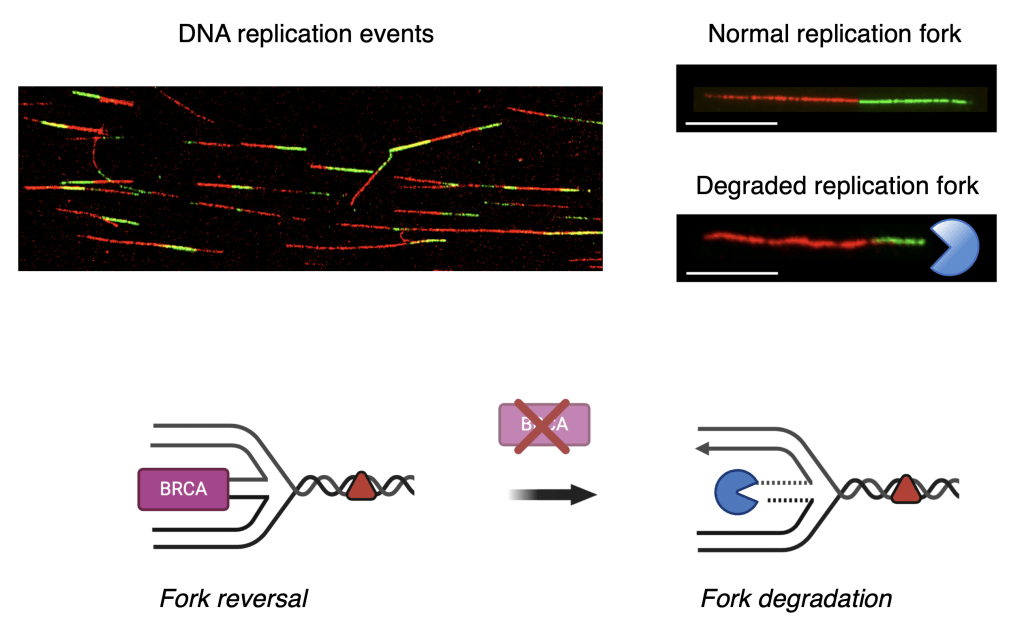
Aside from their well-established roles in homologous recombination, the breast cancer susceptibility proteins BRCA1 and BRCA2 are emerging as key factors required for the maintenance of DNA replication fork stability following chemotherapy. We discovered that the main function of BRCA proteins in the context of replication fork stability is to protect reversed replication forks from nuclease-dependent degradation. When BRCA proteins are missing, the reversed DNA forks are unprotected, and nucleases can easily degrade the DNA. This extensive replication fork degradation phenotype has been linked to the sensitivity of BRCA-deficient tumors to agents that damage the DNA or that inhibit specific repair pathways, such PARP inhibitors. To this end, we also discovered that BRCA-deficient cells employ specific fork recovery pathways as a last resort to rescue the degraded forks and withstand DNA-damaging chemotherapy. Following these findings, our next goal is to elucidate the pathways that control fork recovery in BRCA-deficient tumors, and to understand how to target these pathways for the development of new and more effective cancer treatment strategies. Collectively, our studies revisit the functions of central homologous recombination factors in DNA replication and are crucial to understanding how targeting fork recovery pathways can modulate current chemotherapeutic modalities.
You must be logged in to post a comment.